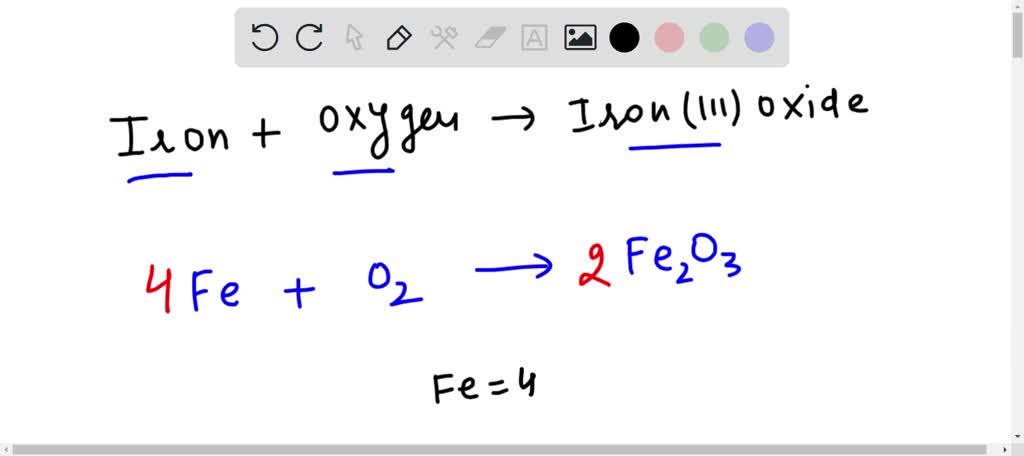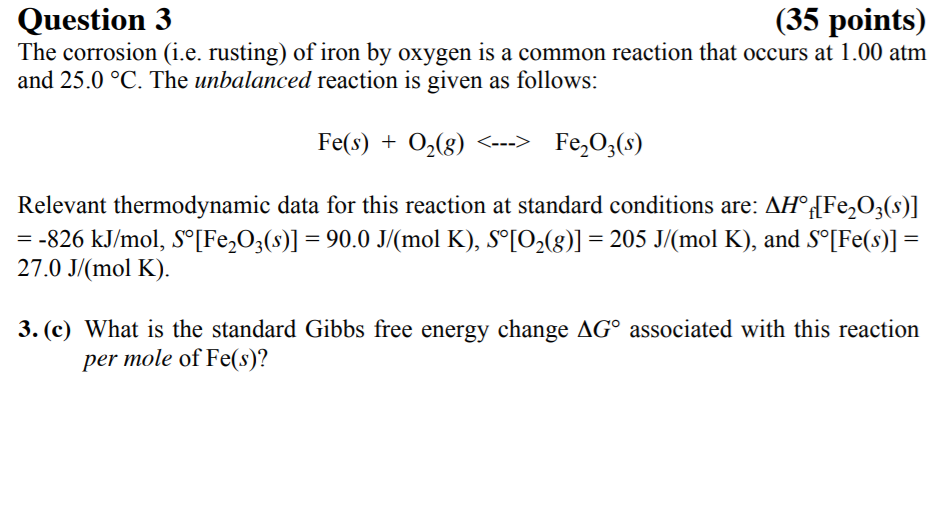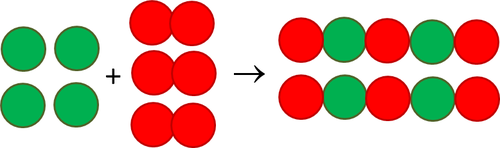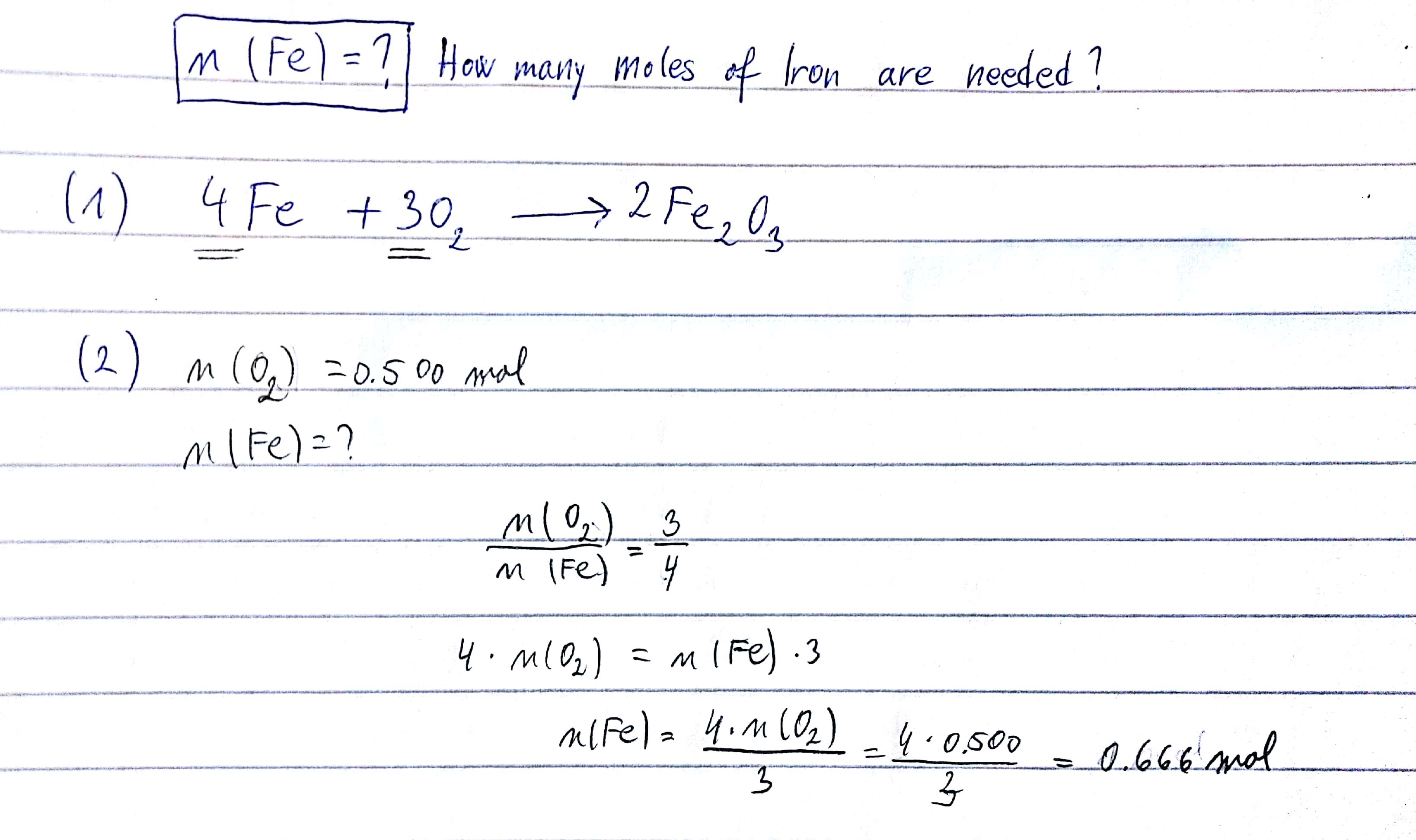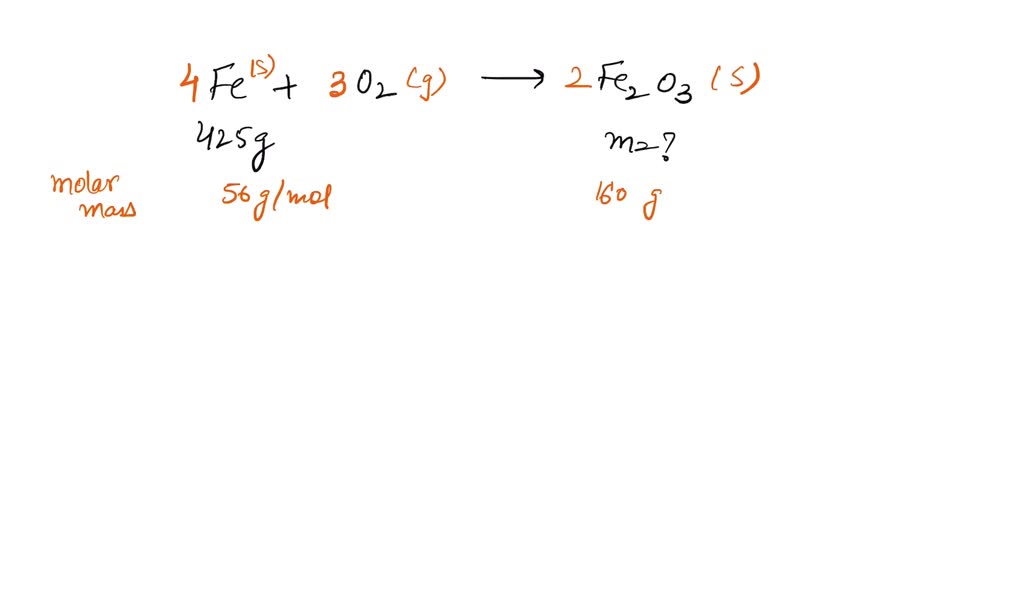
SOLVED: The formation of rust which is iron (III) oxide on the surface of iron metal is an oxidation-reduction reaction between iron metal and oxygen gas. balanced equation : Fe (s) +

January 22, 2016 Learning Target: I will construct a chemical equation for a chemical reaction. Entry Task: All chemical reactions have reactants (inputs) - ppt download

Write the balanced chemical equation of the following word equation.k Iron Pyrites FeS2+Oxygen→Ferric oxide + Sulphur dioxide

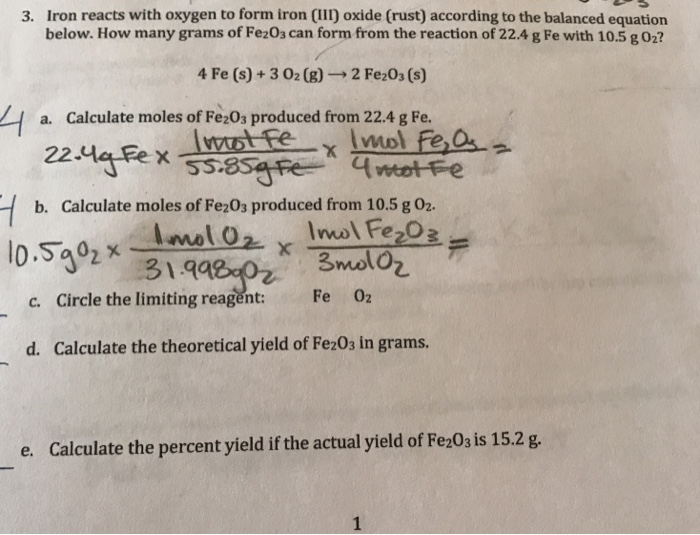
Iron (Fe) reacts with oxygen gas (O2) to form rust (Fe2O3). Balance the equation below by writing in the - brainly.com

Thinkport.org | MPT in the Classroom | Motorweek - Rust (Outermost Electrons and Chemical Reactions)