Stereochemistry of the palladium-catalyzed allylic substitution: the syn-anti dichotomy in the formation of (π-allyl)palladium complexes and their equilibration - ScienceDirect
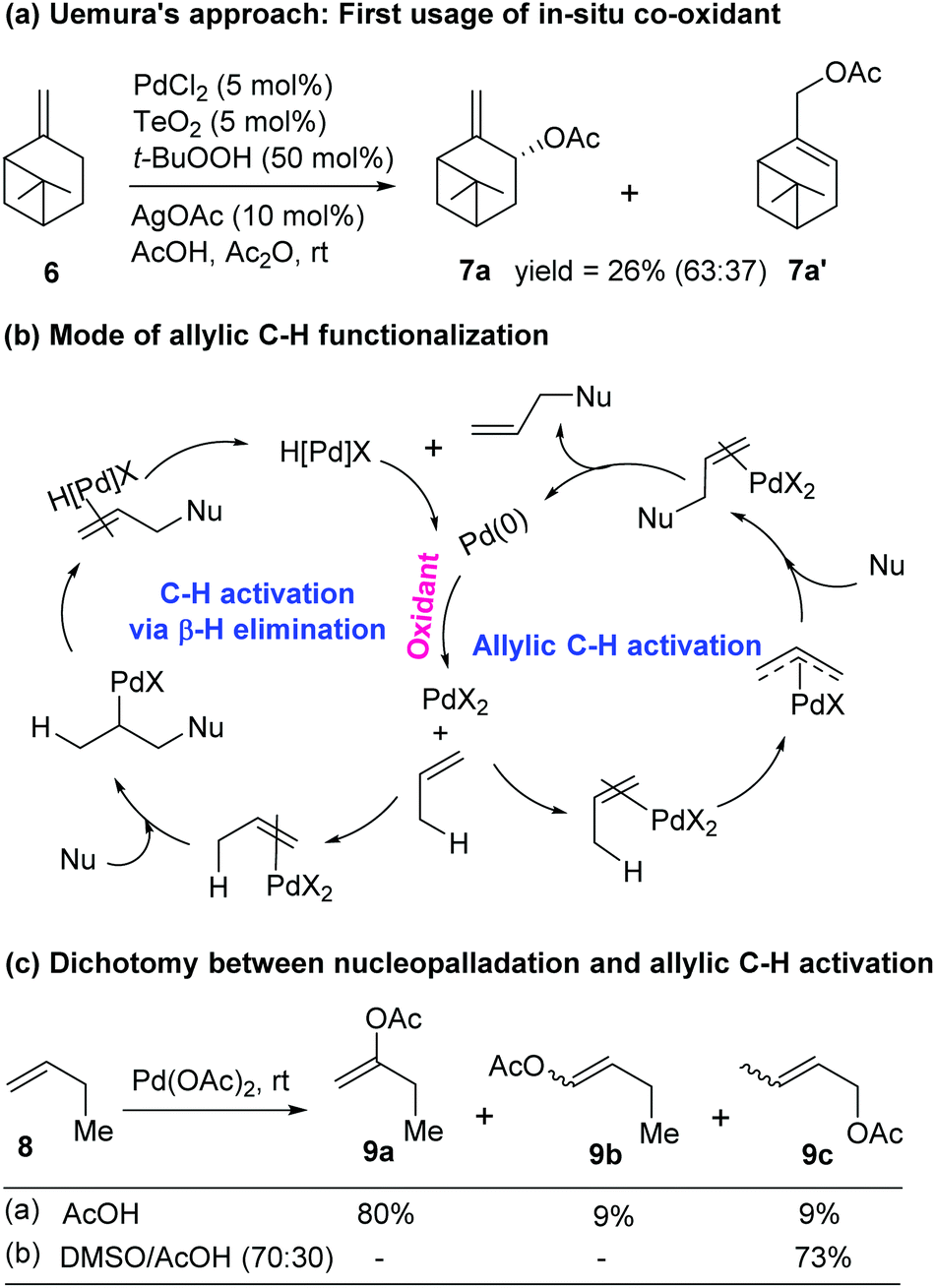
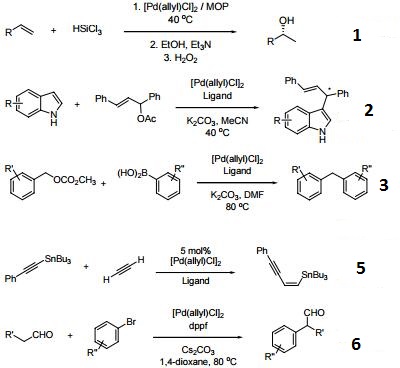
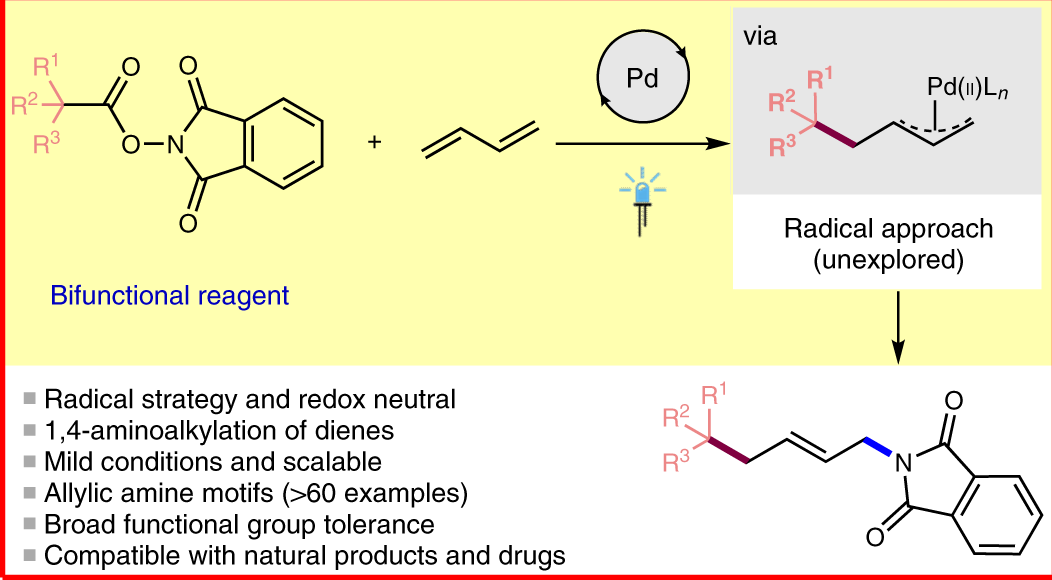
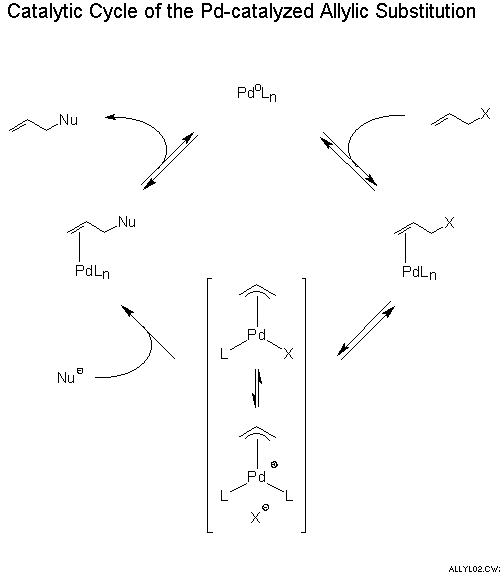
Catalytic allylic functionalization via π-allyl palladium chemistry - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C9OB01725A

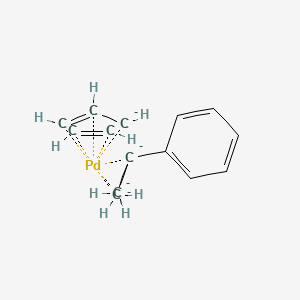
Synthesis and characterization of (π-allyl)palladium(II) complexes containing dialkylbiaryl phosphine ligands - ScienceDirect

Synthesis, characterization, and reactivity of (π-allyl)palladium(II) wrap-around complexes with 1,3-dienes - ScienceDirect
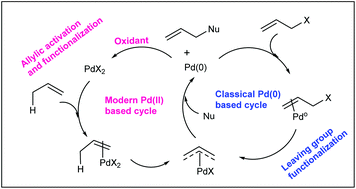
Catalytic allylic functionalization via π-allyl palladium chemistry - Organic & Biomolecular Chemistry (RSC Publishing)

Palladium Enolate Umpolung: Cyclative Diacetoxylation of Alkynyl Cyclohexadienones Promoted by a Pd/SPRIX Catalyst - Takenaka - 2014 - Angewandte Chemie International Edition - Wiley Online Library

Palladium‐Catalyzed Electrophilic Allylation Reactions via Bis(allyl) palladium Complexes and Related Intermediates - Szabó - 2004 - Chemistry – A European Journal - Wiley Online Library

Palladium-Catalyzed Asymmetric Allylic Alkylation/α-Iminol Rearrangement: A Facile Access to 2-Spirocyclic-Indoline Derivatives | CCS Chemistry

Palladium-catalyzed regio- and enantioselective migratory allylic C(sp3)-H functionalization | Nature Communications
![Ligand-controlled regiodivergent π-allyl palladium catalysis enables a switch between [3+2] and [3+3] cycloadditions - Chemical Communications (RSC Publishing) Ligand-controlled regiodivergent π-allyl palladium catalysis enables a switch between [3+2] and [3+3] cycloadditions - Chemical Communications (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/C9CC00611G)
Ligand-controlled regiodivergent π-allyl palladium catalysis enables a switch between [3+2] and [3+3] cycloadditions - Chemical Communications (RSC Publishing)

π-Allyl)palladium Complexes Bearing Diphosphinidenecyclobutene Ligands (DPCB): Highly Active Catalysts for Direct Conversion of Allylic Alcohols | Journal of the American Chemical Society

π-Allyl)palladium Complexes Bearing Diphosphinidenecyclobutene Ligands (DPCB): Highly Active Catalysts for Direct Conversion of Allylic Alcohols | Journal of the American Chemical Society