Teva Issues Voluntary Nationwide Recall of One Lot of Anagrelide Capsules, USP 0.5 mg Due to Dissolution Test Failure

FDA announces recall of platelet-reducing medication due to risk of clotting or other adverse cardiovascular outcomes

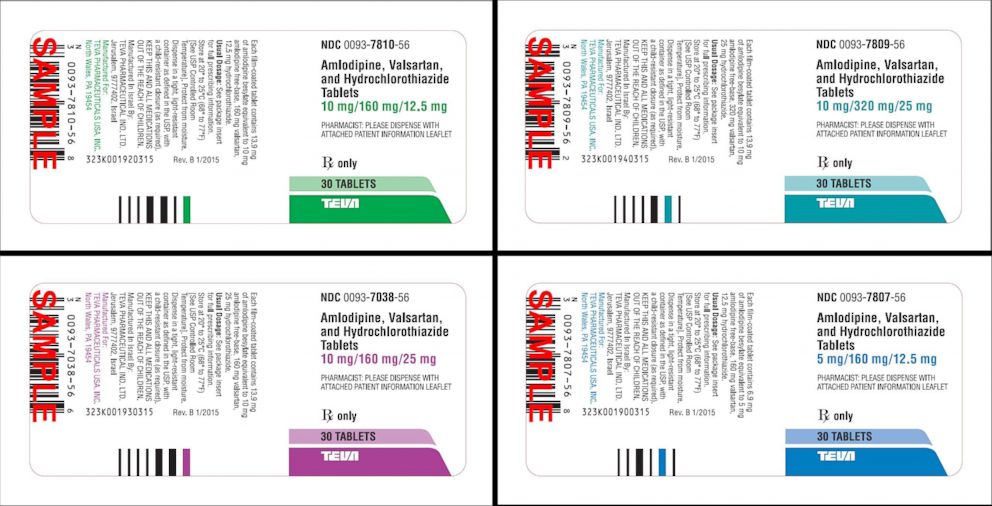
Teva Pharmaceuticals USA, Inc. Initiates Voluntary Nationwide Recall of Metformin Hydrochloride Extended-Release Tablets USP 500 mg and 750 mg Due to Detection of N-Nitrosodimethylamine (NDMA) | FDA

Teva Issues Voluntary Nationwide Recall of One Lot of Anagrelide Capsules, USP 0.5 mg Due to Dissolution Test Failure | FDA
IMPORTANT Metformin Hydrochloride Extended Release Tablets, USP, 500 mg and 750 mg RECALL NOTICE Dear Member, Your health is imp
FDA Safety Alert: Teva Initiates Voluntary Nationwide Recall of Specific Lots of Fentanyl Buccal Tablets CII Due to a Labeling Error - Drugs.com

Teva Initiates Voluntary Nationwide Recall of One Lot of Topotecan Injection 4 mg/4 mL (1 mg/mL) Due to Presence of Particulate Matter | FDA