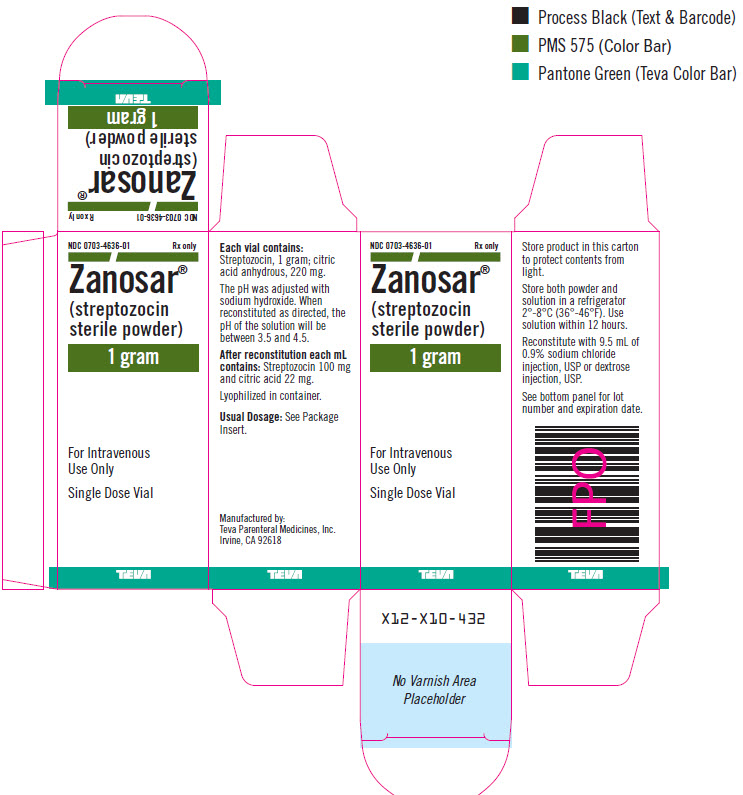
ELI LILLY AND COMPANY, Plaintiff, v. TEVA PARENTERAL MEDICINES, INC., APP PHARMACEUTICALS, LLC, PLIVA HRVATSKA D.O.O., TEVA PHAR
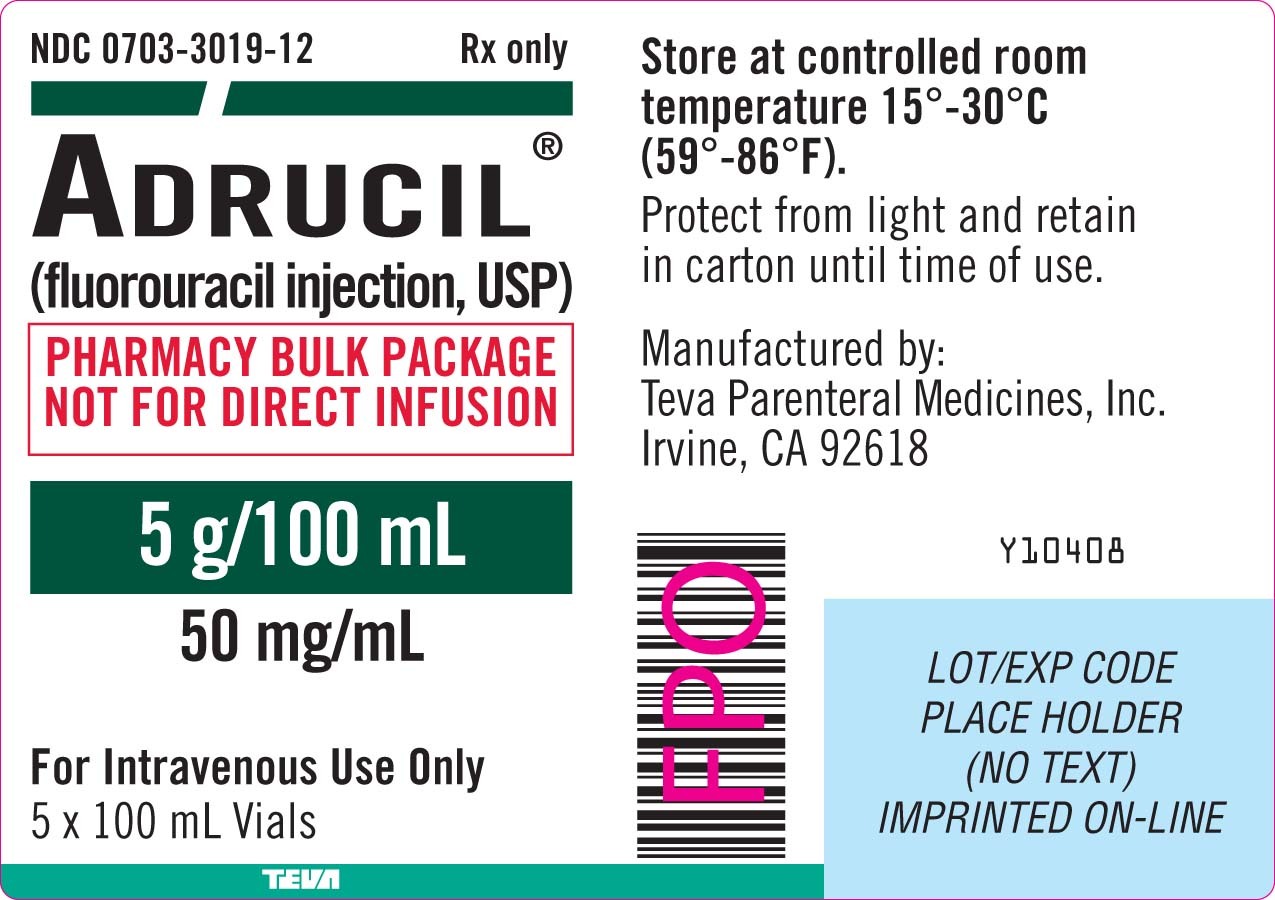
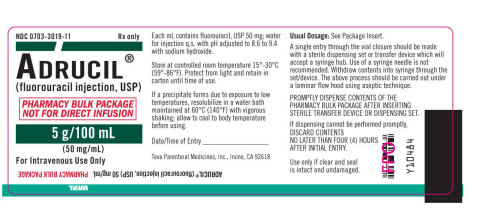
Teva Parenteral Medicines Initiates Voluntary Nationwide Recall of Select Lots of Adrucil® (fluorouracil injection, USP) 5 g/100 mL (50 mg/mL) Due to Particulate Matter | Business Wire

Hsbc Holdings PLC Has $4.77 Million Stock Position in Teva Pharmaceutical Industries Limited (NYSE:TEVA) - American Banking and Market News