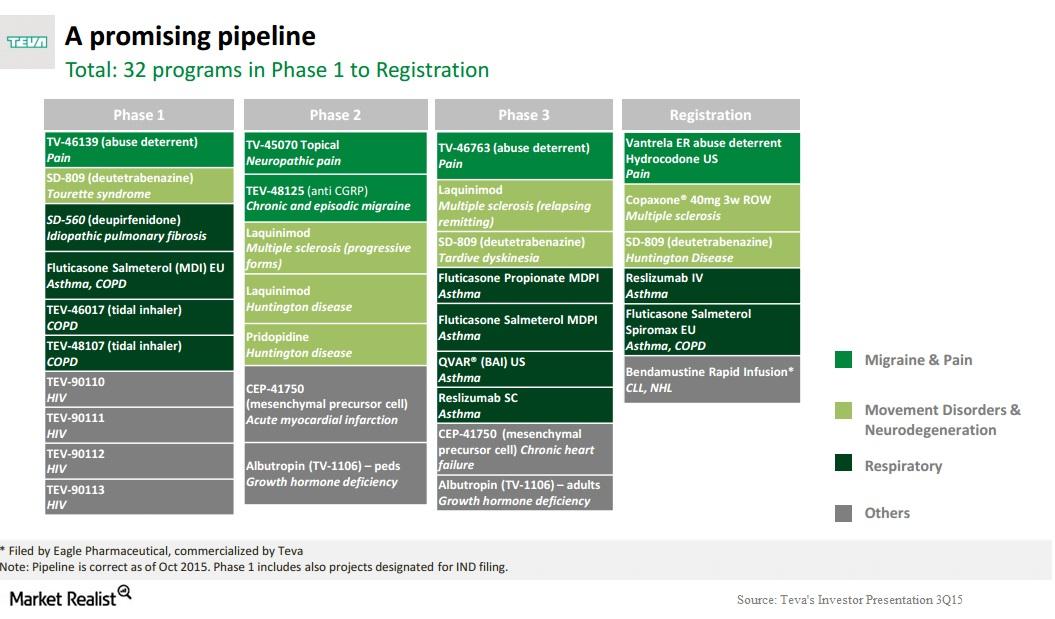
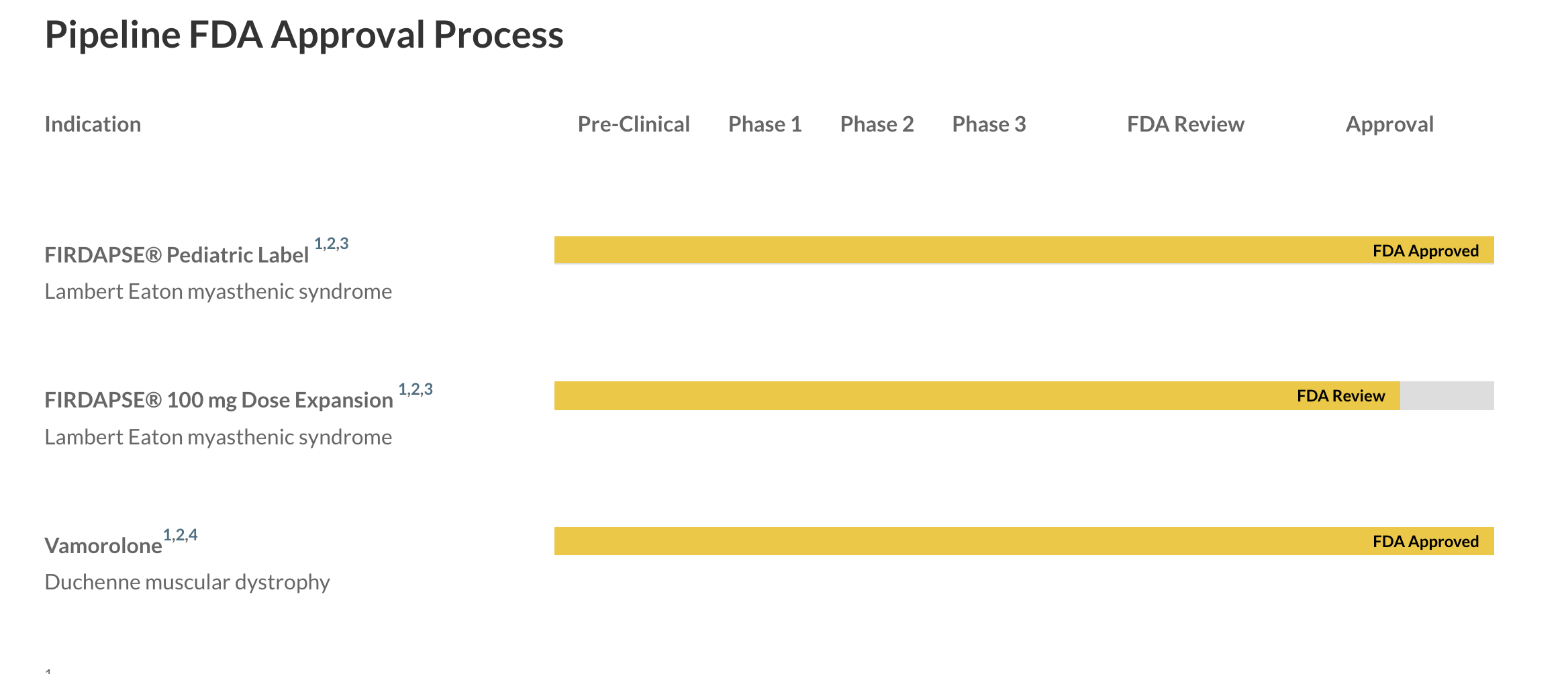
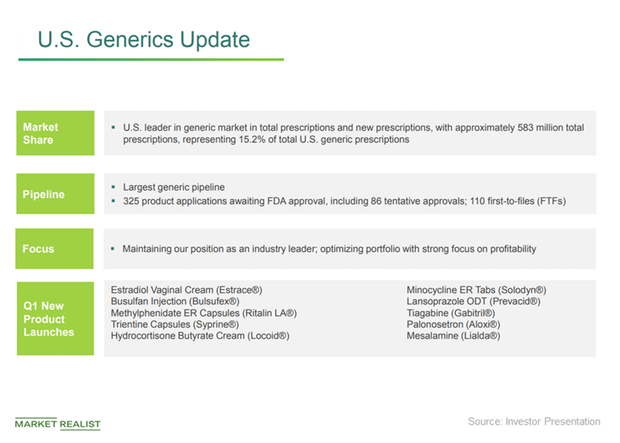
Teva Pharmaceuticals and Alvotech Provide Update on Strategic Biosimilars Partnership | Business Wire

Teva Announces FDA Approval of First and Only Digital Inhaler with Built-In Sensors – ProAir® Digihaler™ | SnackSafely.com
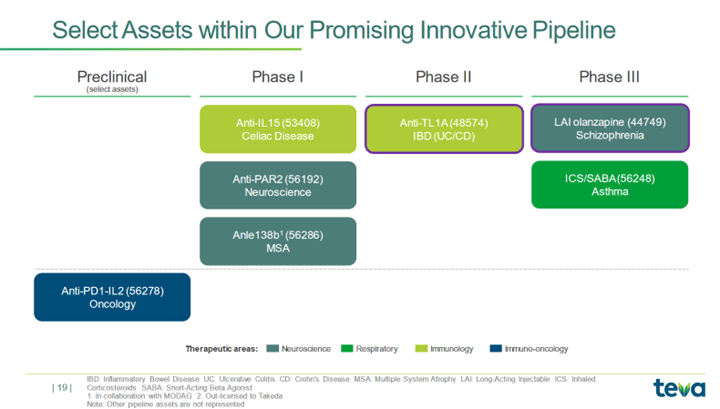
The Mighty Teva Pharmaceutical: Unveiling A Bullish Scenario You Must Consider (NYSE:TEVA) | Seeking Alpha

Alvotech and Teva delay launch of Humira biosimilar as FDA cites manufacturing issues | HealthCare Middle East & Africa Magazine
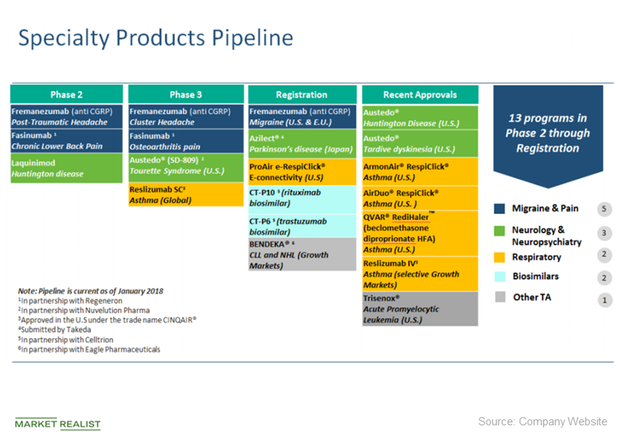
ADDING MULTIMEDIA Teva Announces U.S. Approval of AJOVYTM (fremanezumab-vfrm) Injection, the First and Only Anti-CGRP Treatment with Both Quarterly and Monthly Dosing for the Preventive Treatment of Migraine in Adults | Business

Teva Pharmaceuticals and IBM Expand Global Partnership to Enable Drug Development and Chronic Disease Management with Watson